Click for pdf: Consequences of Prematurity
1. Definition
Prematurity is defined as an underdeveloped newborn child with a low birth weight that is born before 37 weeks of gestation. Other terms used to describe prematurity are: “preterm” and “preemie.” Infants having a gestational age of 35 and 37 weeks are termed ‘moderately premature,’ those born between 29 and 34 weeks of gestation are termed ‘very premature’, and those born at 28 weeks of gestation or less are termed ‘extremely premature.
2. What Gestational Age is Viable?
The survival rate of infant’s born between 22 and 31 weeks of gestation increases with each additional week of gestation. The Textbook of Neonatal Resuscitation suggests that non-initiation of resuscitation for newborns less than 23 weeks gestational age and-or 400 grams in birth weight is appropriate. Most babies born after about 26 weeks gestation do survive to one year, although they may face an extended stay in the NICU.
Improvements of medical technology have increased the viable age for infants with a rising rate of survival for premature infants between 23 – 31 weeks. The following table displays the statistics obtained for the survival of live inborn babies admitted to NICU from 1995 to 2007.
Survival of live inborn babies admitted to NICU from 1995 to 2007 (n = 2334) Adapted from National Women’s Newborn Services Annual Clinical Report (2007)
Infants can also be categorized by birth weight. A low birth weight infant weighs less than 2500g, (5 1/2 lbs) a very low birth weight infant weighs less than 1500g (3 1/2 lbs) and an extremely low birth weight infant weighs less than 1000g (2 1/4 lbs) which helps in determining their true gestational age. “Accurate gestational age is critical because the variation of 1 week in the determined age of an extremely premature infant (25 weeks instead of 24 weeks, for example) produces a far different set of prognostic implications. The initial complete examination at birth is the best way to assess gestational age accurately. In the early minutes to hours of the life of the marginally viable infant, much medical information becomes available and—typically—drives the decision-making process. At this point, predictions are made, outcomes are assessed, and a medical plan is put in place” [17]. The viability limit differs by age from hospital to hospital, although very few babies have survived being born at the gestational age of 22 weeks, viability limits usually fall somewhere between 23 and 35 weeks.
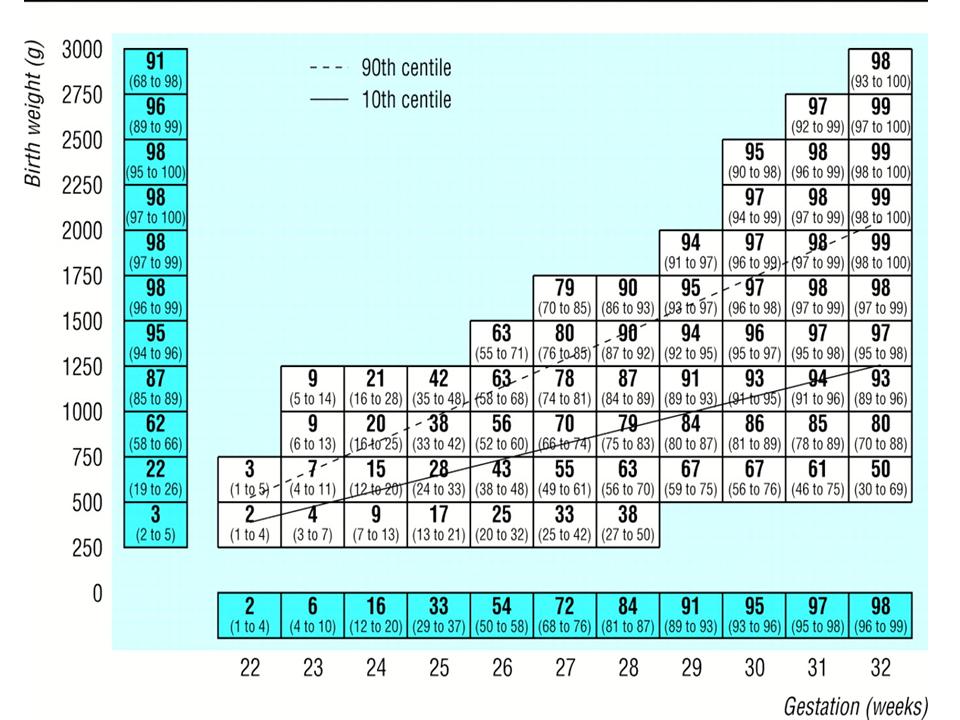
Median (95% confidence interval) predicted percentage survival for European infants known to be alive at onset of labour. Values above 90th centile represent infants large for gestational age, values below 10th centile represent infants small for gestational age. Adapted from Draper et al. (1999).
3. Presentation
Currently, 12 percent of all babies are born premature. This has been a rising statistic as multiple births are now more common, likely as a result of in vitro fertilization. Most premature infants are characterized by low birth weight meaning that they weigh less than 2500 grams (5.5 pounds). Premature infants may also be unable to feed by mouth, breathe without apneas, or thermo-regulate. As a result of being born prematurely, major organs will not have had enough time be fully develop, leading to several premature specific health consequences. Although any premature infant can be born with health risks, infants born before 32 weeks are most likely to have severe health risks.
Possible complications include (see appendix for definition):
Intraventricular Hemorrhage (IVH)
- Respiratory Distress Syndrome (RDS)
- Bronchopulmonary Dysplasia (BPD)
- Anemia of Prematurity
- Neonatal Sepsis and Other Infections
- Congenital Heart Disease
- Hypoglycemia
- Hyperbilirubinemia
- Retinopathy of Prematurity (ROP)
- Necrotizing enterocolitis (severe intestinal inflammation)
- Delayed growth and development
4. What causes premature births?
In approximately 40 percent of premature births, the cause is unknown. However, there are many reasons why a premature birth occurs. Women who have had any of the following are at risk of premature births:
- Having previously delivered prematurely
- Premature rupture of the amniotic sac (ruptured membranes)
- Infections of the urinary tract or cervix
- A weak cervix—prior surgical procedures
- Abnormalities in the uterus, including fibroids and malformations of the
- uterus
- Multiple gestation
- Smoking, drinking, or other substance use during pregnancy
- Poor nutrition during pregnancy
- Polyhydramnios (an excess amount of amniotic fluid)
- Chronic diseases carried by the mother that correlate with premature births include:
- Diabetes
- Heart disease
- Kidney disease
- Systemic Lupus Erythematous
- High blood pressure (Pregnancy Induced Hypertension and HELLP Syndrome)
5. What should family physicians be aware of when seeing these children?
The major medical concerns involving premature infants are the result of under-development of the major organs and primary functions of the human body. The following categories, adapted from Sears et al. (2004), describes the signs, symptoms and possible outcomes for infants who are born prematurely:
Central Nervous System:
Infants born at 32 weeks are highly susceptible to intraventricular hemorrhage (IVH). The upper part of the brain consists of two cerebral hemispheres and within each is a ventricle where cerebralspinal fluid is produced and circulates towards the subarachnoid space. Increased susceptibility results from stressors of other conditions due to prematurity such as respiratory distress. Fluid from both hemispheres flow toward central and lower parts of the brain through narrow channels, and then toward and around the spinal cord. Blood vessels break in the germinal matrix, which is characterized as a small region next to the ventricles where new nerve cells are produced. The germinal matrix is more prominent and fragile in smaller, premature infants and dissapears by approximately 34 weeks whereafter bleeding is unlikely.
The extent of IVH is graded according to the following table:
| Classification | Prognosis | |
| Grade I | Bleeding in the germinal matrix only. (No blood in the ventricles.) | Grade I and II bleeds are reabsorbed and often have no permanent effects. |
| Grade II | Bleeding in the germinal matrix and ventricle(s), without enlargement of the ventricle(s). | |
| Grade III | The quantity of blood in the ventricle(s) is large enough to cause enlargement of the ventricle(s). | Grade III bleeds can cause deficits due to stretching of the brain that occurs when the ventricles are dilated. |
| Grade IV | Blood in extended areas of the brain beyond the germinal matrix. | Usually associated with permanent effects due to the direct damage of brain tissue by the bleeding; The degree of permanent deficit relates to the size and location of bleeding. |
Adapted from University of Pennsylvania Health Systems (2009)
It is very difficult to predict the degree of long-term effects resulting from neonatal brain injury, since most brain development has yet to occur, and significant compensation for deficits can take place.
Ventriculoperitoneal shunts
Used in surgery to relieve pressure inside the skull due to fluid buildup on the brain known as hydrocephalus. This procedure is done by removing fluid from the brain to another part of the body and should be done as soon as hydrocephalus is diagnosed. The procedure is ultimately done to remove excess fluid and reduce pressure in the brain. This procedure involves the use of general anesthesia. A catheter is placed into one lateral ventricle and attached to a cap and valve positioned below the scalp. Tubing is tunneled subcutaneously from the valve to the abdomen, where it is deposited into the sterile peritoneal cavity for continuous drainage. Shunts typically transfer and drain the CSF from the lateral ventricles of the brain and empty the CSF into the right peritoneal cavity. As with any foreign object placed in the body, infection is potentially a dangerous complication.
Periventricular leukomalacia (PVL)
PVL is the most common ischemic brain injury in premature infants. The ischemia occurs in the border zone at the end of arterial vascular distributions and in the white matter adjacent to the lateral ventricles. The diagnostic hallmarks of PVL are periventricular echodensities or cysts detected by cranial ultrasonography. Diagnosing PVL is important due to the significant percentage of surviving premature infants with PVL who develop cerebral palsy (CP), intellectual impairment, or visual disturbances.
Hypoxic Ischemic Encephalopathy (HIE)
HIE is characterized as an acute problem with swelling and irritation of the brain caused by lack of oxygen to the brain. In mild cases the baby recovers completely, however in more severe cases it can result in permanent brain damage. The most common cause of neonatal seizures is HIE brain injury. An asphyxial injury may occur in utero as a result of decreased uteroplacental perfusion, for example in abruptio placenta, cord compression, preeclampsia, or chorioamnionitis. Postnatally, conditions such as persistent pulmonary hypertension of the newborn, cyanotic congenital heart disease, sepsis, and meningitis can also result in hypoxic-ischemic brain injury. In those infants with HIE who have seizures, onset of seizures is generally within the first 24 hours after birth. However, the timing of onset is not a reliable indicator of the timing of the neurologic injury.
Treatment of neonatal seizures should focus on the primary etiology as well as direct seizure control. Phenobarbital is often used as the first line anticonvulsant, followed by phenytoin and lorazepam. Oral phenytoin is poorly absorbed from the infant GI tract. Infants who survive severe encephalopathy are more likely to experience a poor outcome at age 2 years in comparison with infants who recover from moderate encephalopathy (62% vs 25%). Overall, nearly 40% of infants who experience neonatal encephalopathy exhibit significant developmental delay at age 2 years compared with healthy children. Suboptimal head growth occurs in approximately 50% of infants surviving hypoxic-ischemic encephalopathy and is associated with white matter injury and basal ganglia and thalamic lesions. Severe hearing impairment is an important long-term consequence, necessitating sequential surveillance through age 3 years. Children who have 5-minute Apgar scores of 3 or less at birth and signs of neonatal encephalopathy are at increased risk of developing minor motor impairments and seizures. They demonstrate a greater-than-expected need for educational assistance during their early school years and show decreased performance in reading, mathematics, and fine-motor skills. Behavioral and emotional problems are also more prevalent.
Pulmonary System:
Delaying birth gives lung tissue extra time to mature, and improves lung function of premature babies at birth. During the delay before birth, mothers of premature babies may be prescribed steroids. “Steroids (glucocorticoids) can speed the development of a preterm infant’s lungs between 24 and 34 weeks gestation, and are often administered during preterm labor” [6]. Steroids help promote the production of surfactant, a substance that fetus’ lungs start making at around 26 to 34 weeks of pregnancy, coats the insides of the lungs and keeps them open so they can breathe in air after birth; Ultimately, surfactant prevents the collapse of alveoli (small sacs in the lungs where air is exchanged).
The timing of the dose of steroids is important. Steroids must be given to the mother as an injection several hours before the infant is delivered. A second dose is usually given 24 hours after the first dose. There is probably some benefit from steroids, even if the woman delivers before the second dose is given. The greatest benefit is seen when the steroid is given at least 48 hours before the infant is delivered [6]. Currently, the most commonly used steroid is betamethasone, used to decrease the infant’s risk for intraventricular hemorrhage (bleeding into the brain), complications affecting the bowels, circulatory system and a common condition known as respiratory distress syndrome (RDS).
RDS occurs when the infants lungs are not developed enough to make surfactant. The baby then has to work hard to breathe. Signs and symptoms that are seen with RDS include:
- Rapid, shallow breathing
- Sharp pulling on the chest below the ribs with each breath taken in.
- Grunting sounds during exhalation
- Flaring of the nostrils during breathing.
Artificial breathing devices and surfactant can be used to treat this syndrome, however, depending on how severe the RDS is, these babies may develop other serious medical problems that include:
- A collapsed lung
- Leakage of air from the lung into the chest cavity
- Broncopulmonary dysplasia (BDP)
- Intraventricular hemmorage (IVH)
- Sepsis
- Bleeding in the lungs
- Kidney failure
- Necrotizing enterocolitis (NEC)
Another common conditions regarding the pulmonary system of the premature child is known as broncho-pulmonary dysplasia (BPD). This is a chronic lung disease caused by high levels of oxygen for long periods of time or with prolonged treatment of respiratory distress syndrome using a ventilator. Long term consequences consist of chronic lung diseases such as asthma and cystic fibrosis. Further, many become very susceptible to respiratory infections such as influenza, respiratory syncytial virus (RSV), pulmonary edema and pneumonia.
Children who have a history of BPD may develop rare complications that can occur with the ciruculatory system resulting in pulmonary hypertension. Overall, the effects of BPD can play a significant role in regards to increased chest infections and decreased exercise tolerance. Medications needed to inhibit the effects of BPD can even result in undesired effects such as dehydration and low sodium levels from diuretics; kidney stones, hearing problems and low potassium and calcium levels from long term use of furosemide.
Cardiovascular System:
The heart in fully developed infants has a ductus arteriosus (DA) that allows proper uptake of oxygen through the umbilical cord. With the infant’s first breath, this ductus arteriosis constricts to allow the pulmonary circulation to take over. Premature infants can be critically under-developed which can result in patent ductus arteriosus (PDA), which causes abnormal blood flow between the aorta and pulmonary artery, leading to heart failure.
In normal birth weight and full-term neonates, the DA closes within 3 days after birth. However, the DA is patent for more than 3 days after birth in 80% of preterm neonates weighing less than 750 g and its persistent patency is associated with increased morbidity and mortality. Furthermore, in the presence of a significant left-to-right ductal shunt in low birth weight (LBW) neonates, a decreased peripheral perfusion and oxygen delivery occurs. At birth, expansion of the neonatal lungs is associated with an immediate fall in pulmonary vascular resistance. In neonates, a heart murmur is discovered within the first few days or weeks of life. The murmur is usually recognized as systolic rather than continuous in the first weeks of life and can mimic a benign systolic murmur [12].
A hyper dynamic, precordial impulse, full pulses, widened pulse pressure, hepatomegaly, and a high parasternal systolic murmur have been described as the classical physical signs of PDA. They usually appear about day 5 onwards and, together with evidence of interrupted improvement of worsening respiratory status, have been established as the clinical criteria of heamodynamic significance [5].
Gastrointestinal System:
Premature infants lack the full ability to obtain nutritional needs from the placenta via the umbilical cord. They do not have fully developed digestive systems and are incapable of properly processing food. As a result, newborn premature infants are highly susceptible to inflammation of the lining of the intestines as well as many other possible infections including necrotizing enterocolitis – which results in bowel obstruction or tissue death.
Necrotizing enterocolitis (NEC)
NEC is the most common gastrointestinal disorder of premature infants. Despite affecting thousands of neonates in the US alone, the etiology of NEC is unknown and no effective preventative treatments exists. Development of NEC is likely multifactorial, with prematurity, enteral formula feeding, intestinal hypoxia/ischemia and bacterial colonization being the major risk factors [7].
NEC typically occurs within the fist 2 weeks of life and involves many signs and symptoms that may include:
- poor tolerance to feedings
- feedings stay in stomach longer than expected
- decreased bowel sounds
- abdominal distension (bloating) and tenderness
- greenish (bile-colored) vomit
- redness of the abdomen
- increase in stools, or lack of stools
- bloody stools
- apnea
- bradycardia
- diarrhea
- lethargy
- fluctuating body temperature.
- advanced cases may show fluid in the peritoneal cavity, peritonitis, or shock.
Diagnosis of NEC can be confimred by the prescence of an abnormal gas pattern as seen on an X-ray. Characterized by a “bubbly” appearance of gas in the walls of the intestine (“pneumatosis intestinalis”, large veins of the liver, or the presence of air outside of the intestines in the abdominal cavity.
Treatment of NEC includes:
- stopping feeds
- nasogastric drainage
- intravenous fluids or fluid replacement and nutrition
- frequent examinations and X-rays of the abdomen
Eyes:
Retinopathy is common in premature infants due to the under-development of the blood vessels to the retina. As a result, Retinopathy of Prematurity (when the vessels stop growing or grow abnormally causing bleeding in the eye) can occur in most premature infants. Severe cases can result in vision loss, but in some cases can be treated with surgery, laser therapy or may resolve naturally over time.
The American Academy of Pediatrics, the American Association for Pediatric Ophthalmology and Strabismus, and the American Academy of Ophthalmology released a joint statement recommending that initial screening examinations be performed between 4 and 6 weeks of chronological age or 31 and 33 weeks of postconceptional age.
Hearing:
The probability that a baby will suffer from some sort of hearing deficit increases with the degree of prematurity. Full development of the ear (ear drum, eustachian tube etc.) does not fully mature until as late as the 26th week of pregnancy. As a result, hearing loss in premature infants can be due to injury, infection or a congenital defect.
It is extremely important to assess hearing before infants leave the NICU and to follow up for several months afterwards. Without consistent follow up to assess hearing, premature babies can experience a great disadvantage in learning and development. Missed diagnosis of hearing problems can result in significantly worsened symptoms. Ultimately, whether treatment is successful or not, it is always better to know that the problem exists so that other steps to improve their life and learning can be taken. Types of hearing problems include:
Sensorineural hearing loss
Originates in the inner ear and is frequently due to prenatal infections, asphyxia either during or shortly after birth, or genetic factors. Under usual circumstances, sensorineural hearing loss cannot be reverssed medically or surgically. Hearing aid are often administered to minimize the effect of the condition.
Conduction hearing loss
Originates in the middle or outer ear and is caused by obstructions such as wax, fluid or a rupture and/or puncture of the ear drum inhibiting sound from being conducted to the inner ear. Conduction hearing loss can usually be treated medically or surgically.
Growth and Development:
To understand the growth of a premature infant, it is important to recognize the growing progress of a full term infant. Most healthy full term infants gain weight according to the following table:
| Age | Weight gain per day | Weight gain per month |
| One to three months | 30 g | 900 g |
| Four to 12 months | 20 g | 600 g |
Adapted from Landsdown and Walker (1996)
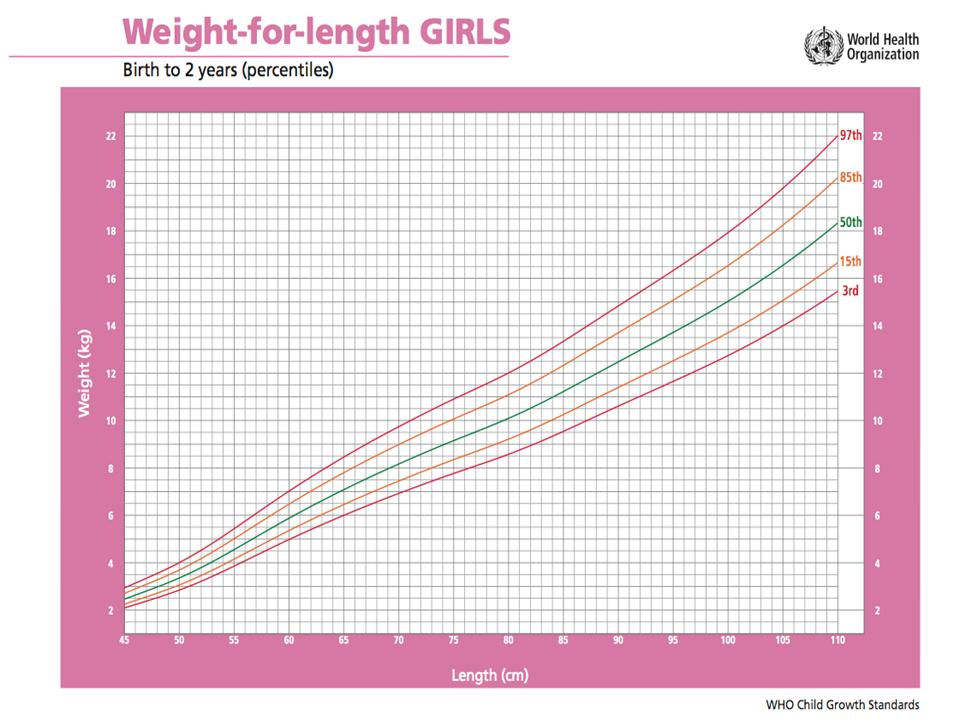
On average, full term newborn babies double their birth weight by four months and triple it by one year of age. The following growth charts, adapted from the World Health Organization (WHO), is useful to compare the progress of the premature baby to a full-term baby.
Many infants, born pre-term or full-term, grow and gain weight at different rates. If inadequate rate of growth becomes prominent using the standard growth chart, it could mean there is a problem such as “failure to thrive”.
Failure to thrive:
- weight less than the third percentile on a standard growth chart
- weight 20% below the ideal weight for height
- fall off from a previously established growth curve.
It is important to carefully assess babies who are failing to thrive and to diagnose a cause for growth failure. Peadiatricians or family doctors must pay particularly close attention to babies experiencing failure to thrive and to follow up on a consistent basis to diagnose the condition.
Brain growth can be dictated by head circumference. Measurements are made at the largest area of the head, above the eyebrows and ears, and around the back of the head. Meausurements obtained should be compared with normal ranges for babies of the same age. Signs to be aware of are an unusually large head which may infer an increased amount of fluid within the skull. A smaller head size may indicate under-development of the brain.
6. What happens to children who survive?
Premature children who survive past the first year of life will have decreased mortality rates compared to full term children. Research done by Swamy et al (2008) found that sixty percent of babies born at 26 weeks of gestation have long-term disabilities that include chronic lung disease, deafness, blindness and neurodevelopment problems. Infants born at 31 weeks were found to be 30 percent less susceptible to these conditions.
Swamy et al. (2008) found that premature children who survive into adolescence continue to demonstrate consequences of their early entry into life. Males born between 22 and 27 weeks were 76 percent less likely to reproduce, whereas women born during the same gestational period were 67 percent less likely to have children. Research also revealed that women who are born prematurely are at much higher risk of giving birth to preterm offspring as well, however, the study revealed men showing no signs of premature successors.
Adapted from Wood et al. (2000).
7. Conclusion
Most premature infants exhibit long term effects of health risks in which many of the medical problems encountered as a result of a premature birth continue into childhood and can remain over the lifespan. Current evidence has found that the more premature an infant, the smaller the birth weight. Although research has provided evidence of long term increased health risks, it is important to keep in mind that the consequences of prematurity vary with each individual. It is our role as physicians to know the health risks that accompany prematurity and follow patients accordingly.
8. Appendix
Anemia of Prematurity – Infants born prematurely develop the anemia of prematurity (AOP), associated with the earlier onset of a more pronounced anemia that is inversely proportional to the gestational age at birth. typically occurs at 3 to 12 weeks after birth in infants less than 32 weeks gestation and resolves spontaneously by three to six months. Many infants are asymptomatic despite having very low concentrations of hemoglobin. Other infants with AOP are symptomatic with tachycardia, poor weight gain, increased requirement of supplemental oxygen, or increased episodes of apnea or bradycardia.
Bronchopulmonary Dysplasia (BPD) – a chronic lung disorder that is most common among children who were born prematurely, with low birthweights and who received prolonged mechanical ventilation to treat respiratory distress syndrome. BPD is clinically defined as oxygen dependence to 21 post-natal days. BPD is characterized by inflammation and scarring in the lungs. More specifically, the high pressures of oxygen delivery result in necrotizing bronchiolitis and alveolar septal injury, further compromising oxygenation of blood.
Heart Disease – Any disorder that affects the heart. Sometimes the term “heart disease” is used narrowly and incorrectly as a synonym for coronary artery disease. Heart disease is synonymous with cardiac disease but not with cardiovascular disease which is any disease of the heart or blood vessels.
Hyperbilirubinemia – An elevated level of the pigment bilirubin in the blood. A sufficient elevation will produce jaundice. Some degree of hyperbilirubinemia is very common in babies right after birth, especially premies.
Hypoxic Ischemic Encephalopathy (HIE) leading to cognitive or motor disability or delay – Damage to cells in the central nervous system (the brain and spinal cord) from inadequate oxygen. Hypoxic-ischemic encephalopathy allegedly may cause in death in the newborn period or result in what is later recognized as developmental delay, mental retardation, or cerebral palsy.
Hypoglycemia – Low blood sugar (glucose). When symptoms of hypoglycemia occur together with a documented blood glucose under 45 mg/dl, and the symptoms promptly resolve with the administration of glucose, the diagnosis of hypoglycemia can be made with some certainty.
Hypoglycemia is only significant when it is associated with symptoms. The symptoms may include anxiety, sweating, tremor, palpitations, nausea, and pallor. Hypoglycemia also starves the brain of glucose energy, which is essential for proper brain function. Lack of glucose energy to the brain can cause symptoms ranging from headache, mild confusion, and abnormal behavior, to loss of consciousness, seizure, and coma. Severe hypoglycemia can cause death.
Intraventricular Hemorrhage (IVH) – bleeding inside or around the ventricles, the spaces in the brain containing the cerebral spinal fluid.
Necrotizing enterocolitis (severe intestinal inflammation) – a serious bacterial infection in the intestine, primarily of sick or premature newborn infants. It can cause the death (necrosis) of intestinal tissue and progress to blood poisoning (septicemia). It is a serious infection that can produce complications in the intestine itself—such as ulcers, perforations (holes) in the intestinal wall, and tissue necrosis—as well as progress to life-threatening septicemia. Necrotizing enterocolitis most commonly affects the lower portion of the small intestine (ileum). It is less common in the colon and upper small bowel.
Neonatal Sepsis – A serious blood bacterial infection in an infant less than 4 weeks of age. Babies with sepsis may be listless, overly sleepy, floppy, weak, and very pale.
Respiratory Distress Syndrome (RDS) – Illness most commonly seen in preemies when the tiny air sacs in the lungs collapse when the baby exhales. It is caused by a lack of lung surfactant.
Retinopathy of Prematurity (ROP) – a disease that affects immature vasculature in the eyes of premature babies. It can be mild with no visual defects, or it may become aggressive with new blood vessel formation (neovascularization) and progress to retinal detachment and blindness. As smaller and younger babies are surviving, the incidence of ROP has increased.
Systemic Lupus Erythematous (SLE) – a chronic inflammatory disease of unknown cause that affects multiple organ systems. Immunologic abnormalities, especially the production of a number of antinuclear antibodies, are another prominent feature of this disease.
References
1. Bradford, N. Your premature baby; the first five years. Firefly Books Ltd, Toronto, Ontario 2003.
2. Braner D., Kattwinkel J., Denson S., Zaichkin J.(2000) American Academy of Pediatrics. Special considerations. Textbook of Neonatal Resuscitation. 4th ed. Elk Grove Village, IL: 2000: 7–19
3. Deborah E. Campbell, MD; Sonia O. Imaizumi, MD; Judy C. Bernbaum, MD. Chapter 95: Health and Developmental Outcomes of Infants Requiring Neonatal Intensive Care. AAP Textbook of Pediatric Care, 2008.
4. Draper, E.S., Manktelow, B., Field, D.J., James, D. (1999). Prediction of survival for preterm births by weight and gestational age: retrospective population based study. BMJ, 319: 1093-1097
5. Evans, N. (1993) Diagnosis of patent ductus arteriosus in the preterm newborn. Archives of disease in childhood: vol. 68 (1 Spec No.): 58-61.
6. Funai, E. Preterm Labour. Uptodate 2007.
7. Halpern, M.D., Holubec, H., Dominguez, J.A., Meza, Y.G., Williams, C.S., Ruth, M.C., McCuskey, R.S., and Dvorak, B. (2003). Hepatic inflammatory mediators contribute to intestinal damage in necrotizing enterocolitis. American Journal of Physiology – Gastrointestinal Liver Physiology: 284: (4)
8. Hutchinson, A.K., Saunders, R.A., O’Neil, J.W., Lovering, A., Wilson, M.E. (1998). Time of Initial Screening Examinations for Retinopathy of Prematurity. Archive of Opthamology: 116 (5): 608-612.
9. Jones H.P, Karuri, S, Cronin, C.M, Ohlsson, A, Peliowski, A, Synnes, A, Lee, S.K. 2005. Actuarial Survival of a Large Canadian Cohort of Preterm Infants. Journal of BMC Pediatrics, 5 (40): 1-13
10. Lansdown, R, Walker, M. Your Child’s Development from Birth to Adolescence. Frances Lincoln Limited, 1996.
11. Lynn M. Iwamoto. Case Based Pediatrics For Medical Students and Residents Department of Pediatrics. University of Hawaii John A. Burns School of Medicine Chapter III.9. Neonatal Seizures
12. Milliken, J.C, D’Souza, G. Patent Ductus Arteriosus. Emedicine, 2007.
13. National Women’s Newborn Services Annual Clinical Report. The Auckland District Health Board, 2007.
14. Sears, W, Sears, R, Sears, J, Sears, M. (2004). The Premature Baby Book: Everything you need to know about your premature baby from birth to age one. Little,Brown and Company, New York, NY.
15. Simpkins, C.J. (2004). Ventriculoperitoneal Shunt Infections in Patients with Hydrocephalus. Pediatric Nursing: 31(6).
16. Swammy, G.K, Ostbye, T, Skjaerven, R. 2008. Association of Preterm Birth with Long-term Survival, Reproduction, and Next-generation Preterm Birth. Journal of the American Medical Association, 299 (12): 1429-1436
17. The University of Pennsylvania Health Systems. Intraventricular Hemmorage. 2009.
18. Verrees, M., Selman, W.R. (2004). Management of Normal Pressure Hydrocephalus. American Family Physician: 70(6).
19. Yates Jr., F.,D. (2008) Medical Decision Making for the Marginally Viable Infant. American Medical Association Journal of Ethics: 10 (10): 673-676.
Acknowledgments
Written by: John Hilhorst
Edited by: Anne Marie Jekyll





 (23 votes, average: 4.61 out of 5)
(23 votes, average: 4.61 out of 5)